
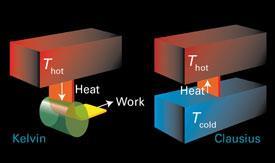
It was his surprisingly simplistic conclusion: if the final state is random, the initial system must have been the opposite, i.e., ordered. ‘Disorder’ in Thermodynamic Entropy Boltzmann’s sense of “increased randomness” as a criterion of the final equilibrium state for a system compared to initial conditions was not wrong. Entropy, S, is a thermodynamic quantity that is a measure of the randomness or disorder or the available arrangements for the system or surroundings.Necessary conditions of equal temperature, equal chemical potential and equal pressure are the consequence of stable equilibrium within the composite system-. Entropy and the Second Law of Thermodynamics The second law of thermodynamics addresses questions about spontaneity in terms of a quantity called entropy.
...
Blackwell Scientific Publications.Chemistry End of Chapter Exercises In Figure 2 all possible distributions and microstates are shown for four different particles shared between two boxes. Compendium of Chemical Terminology, 2nd ed. A microstate is one of the huge number of different accessible arrangements of the molecules' motional energy* for a particular macrostate.Cite as: IUPAC.
The freedom in that part of the universe may increase with no change in the freedom of the rest of the universe. Statistical Entropy - Mass, Energy, and Freedom The energy or the mass of a part of the universe may increase or decrease, but only if there is a corresponding decrease or increase somewhere else in the universe. Qualitatively, entropy is simply a measure how much the energy of atoms and molecules become more spread out in a process and can be defined in terms of statistical probabilities of a system or in terms of the other thermodynamic quantities. Statistical Entropy Entropy is a state function that is often erroneously referred to as the 'state of disorder' of a system. Phase Change, gas expansions, dilution, colligative properties and osmosis. Simple Entropy Changes - Examples Several Examples are given to demonstrate how the statistical definition of entropy and the 2nd law can be applied.


 0 kommentar(er)
0 kommentar(er)
